Showcasing our blood cancer research - BCI at ASH 2021
In December each year, the American Society of Hematology (ASH) hosts its Annual Meeting and Exposition - the premier event in malignant and non-malignant haematology. The event represents an invaluable opportunity for researchers at Barts Cancer Institute (BCI), Queen Mary University of London to highlight their blood cancer research on an international stage.

This year, the 63rd ASH Annual Meeting and Exposition ran from 11-14th December as a hybrid event, with a mixture of virtual and in-person (hosted at the World Congress Center in Atlanta, Georgia) sessions covering key topics in basic and clinical haematology research. Scientific Workshops were held on 10th December.
Researchers from BCI’s Centres for Haemato-Oncology and Cancer Genomics & Computational Biology delivered a variety of oral and poster presentations, and participated in Scientific Workshops.
Professor John Gribben, Lead for BCI’s Centre for Haemato-Oncology, said:
“This was the first live face-to-face event we have had at the international level for the haematology community in two years. More than 13,000 attendees were in Atlanta and with another estimated 25,000 joining online in the hybrid format. For such a large audience it was great to see Barts Cancer Institute so well represented in presentations.”
Professor Gribben chaired and gave a presentation on Targeted Therapies at the Chronic Lymphocytic Leukaemia Society Symposium on 10th December, and co-chaired with the ASH President at a session on real world evidence and its use in haematological malignancies. Professor Gribben presented on the Harmony Big Data platform, which now holds data on more than 98,000 patients with blood cancers throughout Europe.
Targeting altered metabolism in blood cancer
Group Leader, Dr Paolo Gallipoli and postdoctoral researcher, Dr Keith Woodley presented some novel data on a new putative therapeutic target for a very aggressive subtype of acute myeloid leukaemia (AML). The work highlights how a metabolic process within AML cells, called mannose metabolism, can be harnessed to kill AML cells while sparing normal bone marrow cells. Although future work is now required to develop specific inhibitors to target this process, the data highlight the role of this pathway and aberrant metabolism as a therapeutic vulnerability in AML.
Dr Gallipoli presented the work at the Scientific Workshop on Myeloid Development on 10th December, and Dr Woodley presented the findings as an oral presentation on 12th December.
Dr Annalisa D'Avola, postdoctoral researcher in Dr John Riches’ group, delivered an oral presentation on 13th December about their work which has identified a novel role for a metabolic pathway, called the serine synthesis pathway, in the formation germinal centres (structures where white blood cells, called B cells, are activated for the immune response). The serine synthesis pathway and germinal centre formation is controlled by a gene called MYC, which is commonly mutated in lymphoma.
The team found that targeting an enzyme, called PHGDH, in the serine synthesis pathway was able to reduce the formation of germinal centres and the production of antibodies. In addition, they also found that targeting PHGDH impaired cell growth and increased cell death in lymphoma cell lines and blocked the progression of lymphoma in a pre-clinical model. The work represents the first report of the role of the serine synthesis pathway in the biology of the germinal centre response and lymphomas derived from germinal centre B cells. The findings establish PHGDH as a critical player in the immune response and as a clinically relevant target in MYC-driven lymphoma, which is an area of significant unmet need.
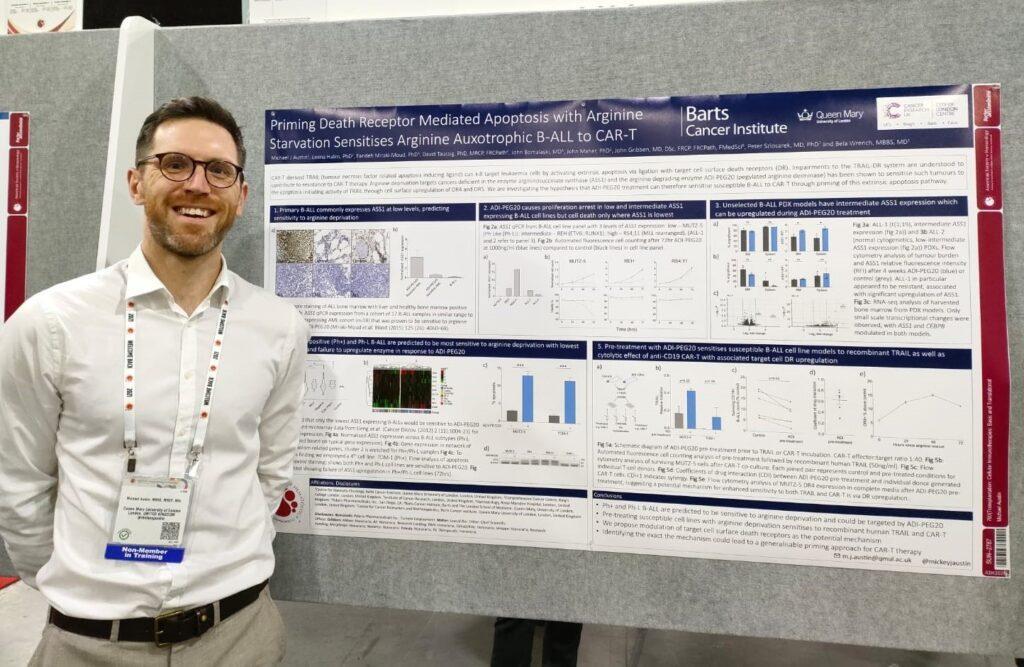
Michael Austin, Clinical Research Fellow in Dr Bela Wrench’s group, presented a poster on research into the dependence of B-cell acute lymphoblastic leukaemia on the amino acid arginine, and whether this dependency could be exploited to render cancer cells more sensitive to immunotherapy.
Novel insights into impaired blood cell production

Group Leader, Dr Kevin Rouault-Pierre and postdoctoral researcher, Dr Celine Philippe presented data that they recently obtained in the laboratory about mutations that occur in a rare type of blood cancer called myelodysplastic syndrome (MDS). One of the main symptom of MDS is anaemia, where red blood cell numbers are reduced because the red blood cell production process (known as erythropoiesis) in the bone marrow is impaired.
Using a cohort of 42 primary human MDS patient samples, the team found that MDS cells that harbour mutations in a gene called SF3B1 consequently have expression errors of a protein known as coenzyme A synthase (COASY). Errors in COASY result in depletion of a key molecule (succinyl-coA) needed for the production of red blood cells. The research reveals, for the first time, a novel critical role of COASY in regulating normal red blood cell production in the bone marrow through control of succinyl-coA availability. Interestingly, the team found that the effects of COASY loss on red blood cell production can be reversed by treating MDS cells with Vitamin B5 or succinyl-CoA.
Dr Rouault-Pierre presented the work at the Scientific Workshop on Stem Cells on 10th December, and Dr Philippe presented the findings as an oral presentation on 11th December.
Dr Ana Rio-Machin, postdoctoral researcher in Professor Jude Fitzgibbon’s group, was invited to chair a session of the Scientific Workshop on Germline Predisposition to Hematopoietic Malignancies and Bone Marrow Failure on 10th December. Dr Hannah Armes, also a postdoctoral researcher in Professor Fitzgibbon’s group and co-supervised by Dr Rouault-Pierre, gave an oral presentation at the same workshop about the effects of mutations in a gene called ERCC6L2 on normal blood cell production (haematopoiesis) in the bone marrow.
ERCC6L2-deficiency is a rare but life-threatening inherited condition that gives rise to bone marrow failure, MDS and AML; however, the consequences of ERCC6L2 loss on normal haematopoiesis and on the bone marrow remain poorly described. By developing cellular models lacking ERCC6L2, the team demonstrated for the first time that ERCC6L2-deficiency results in significant defects in the growth of blood stem cells and their ability to generate the different types of blood cell types required in the body. The research found that red blood cell production is particularly impaired and that the bone marrow microenvironment exhibits significant changes in response to ERCC6L2 loss.
More information
- Find out more about the research in BCI’s Centre for Haemato-Oncology
- Find out more about the research in BCI’s Centre for Cancer Genomics & Computational Biology
- BCI at the 60th ASH Annual Meeting
- ASH 2019 - Our blood cancer research on an international stage
Category: Events, General News

No comments yet