BCI and KCL collaboration develops a clinically-relevant CAR T cell imaging system

A collaboration involving researchers from BCI’s Centre for Molecular Oncology, led by Dr Jane Sosabowski, and the ImmunoEngineering Group of King’s College London (KCL), led by Dr Sophie Papa, has developed an effective and clinically-relevant imaging system to monitor chimeric antigen receptor (CAR) T cells within the body. This system reduced the tumour burden in a pre-clinical model of prostate cancer and allowed for repeated and non-invasive assessment of CAR T cell localisation.
How does CAR T cell therapy work?
CAR T cell therapy- a type of immunotherapy- utilises T cells from patients, which have a critical role in the immune response by killing infected cells. T cells are initially isolated from blood samples of patients and modified in the laboratory to make them express special protein receptors on their surface, called CARs. These CARs recognise specific proteins, known as antigens, on the surface of cancer cells. The modified T cells, now known as CAR T cells, are then multiplied and infused back into the patient where they recognise, target and kill cancer cells expressing the target antigen.
Monitoring CAR T cell behaviour
Dr Sosabowski said:
CAR T cell therapy is being used successfully for the treatment of some blood cancers; however, solid tumours have proved much more difficult to treat in this way. The ability to image and track the T cells as they migrate around the body would greatly facilitate this area of research.
The present findings, published in Nature Communications, characterise a system that enables this.
In the study, funded by grants from the Medical Research Council, T cells from healthy volunteers were modified to express a CAR that recognises prostate-specific membrane antigen (PSMA)- a protein expressed on the surface of prostate cancer cells.
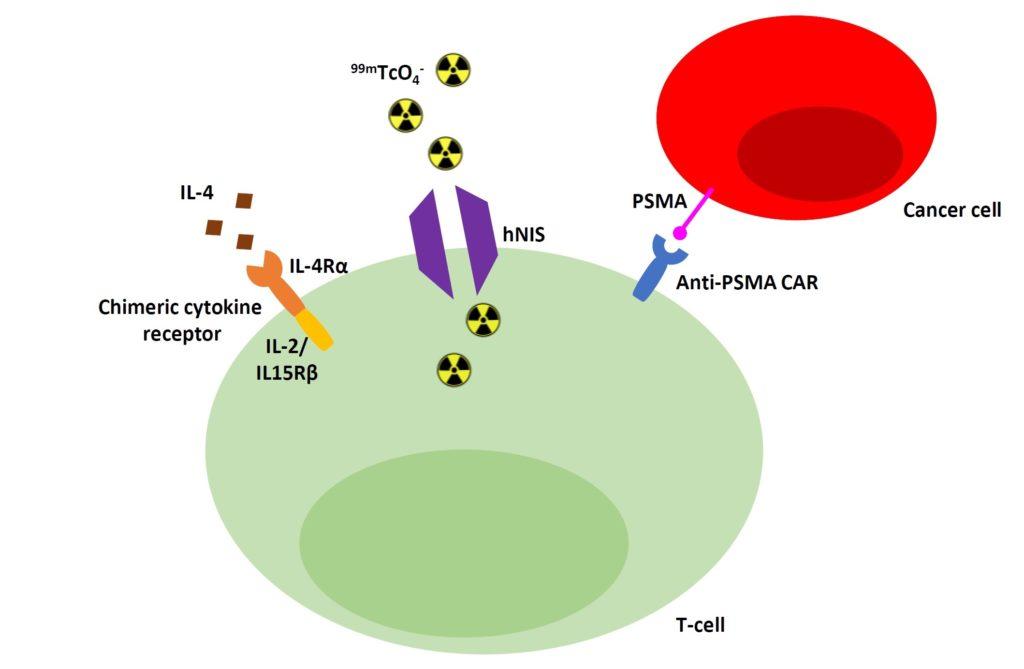
The cells were also engineered to express human sodium iodide symporter (hNIS). hNIS is a protein that acts as a channel in the cell membrane, allowing live cells to take up 99mTcO4- , a commonly used radioactive tracer that emits a detectable signal. In this way, uptake of 99mTcO4- by the CAR T cells means they can be visualised using a specialised imaging system, revealing their location when inside the body.
An effective system
In order to determine the effectiveness of the system, the CAR T cells were injected into a pre-clinical model with PSMA-positive prostate cancer tumours and the model was imaged 7, 14 and 21 days later after injection of 99mTcO4- at each time point.
The CAR targeted the T cells to the tumours and rapid CAR T cell accumulation at the tumour site was observed, indicated by detection of 99mTcO4- . By day 21, injection of 99mTcO4- showed no signal at the tumour site, reflecting loss of T cells from the site and eradication of the tumours.
Notably, injection of CAR T cells that had been modified to remove their killing capabilities were able to migrate to the tumours; however, 99mTcO4- was still detected at day 21, indicating that these T cells were unable to eradicate the tumour and control the disease.
Dr Papa said:
Being able to visualise the failure of the therapy as well as the success is particularly valuable for clinical application. If the CAR T cell treatment is not working or is causing toxicity in the patient, the ability to identify this at an early stage will aid decision making for a treatment regimen, preventing patients from being treated with ineffective or harmful agents.
Future directions
The team hope that this system will be adopted for treatment in humans, providing a non-invasive tool for repeated monitoring of CAR T cell therapies in patients. T cells can be modified to express different CARs, and so this system could be applied to monitor this therapy in different cancer types. In the future, the team endeavour to use 99mTcO4- signalling to quantify the number of CAR T cells that are present at the tumour site.
Category: General News, Publications

No comments yet