IBD and bowel cancer: Predicting patient risk
Bowel Cancer Awareness Month
 April is Bowel Cancer Awareness Month. We recently spoke with Dr Kit Curtius, UKRI Rutherford Research Fellow, about her work which focuses on understanding how normal tissues evolve to become cancerous. Dr Curtius is particularly interested in gastrointestinal pre-malignancies such as inflammatory bowel disease (IBD), which may increase an individual’s risk of developing bowel cancer.
April is Bowel Cancer Awareness Month. We recently spoke with Dr Kit Curtius, UKRI Rutherford Research Fellow, about her work which focuses on understanding how normal tissues evolve to become cancerous. Dr Curtius is particularly interested in gastrointestinal pre-malignancies such as inflammatory bowel disease (IBD), which may increase an individual’s risk of developing bowel cancer.
Bowel cancer, also known as colorectal cancer (CRC), is the fourth most common cancer in the UK, with approximately 41,700 new cases diagnosed each year. Unfortunately, about 16,000 people lose their lives to this cancer type each year in the UK, making it the second most common cause of cancer mortality.
Kit, what is the likelihood of someone with IBD developing CRC?
Nearly 20% of patients with IBD are expected to develop CRC in their lifetime. This is four times higher than the risk of CRC in the average population. Identifying those particular patients who are part of that proportion of high risk individuals and recommending preventative action is the challenge we face.
Could you tell us about your research and what you ultimately hope to achieve?
My research focuses on understanding the evolutionary dynamics governing the transition from normal tissue to malignant tissue in human patients. In my experience, this type of study requires interdisciplinary expertise - from cellular biology, to evolutionary genetics, to population health data science. My work integrates multi-scale data within mathematical models that I ultimately hope will inform cancer screening recommendations and improve precision medicine.
What information do you use to predict cancer risk?
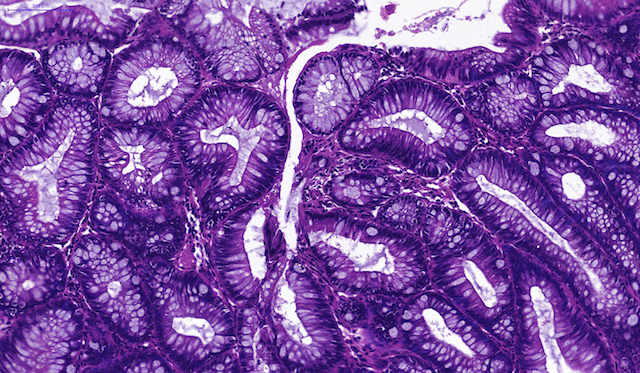
Ideally, we aim to use as much information as feasible about a patient to understand disease status and make future predictions about cancer risk. The critical gap to bridge is our understanding of the hidden relationships between macro-scale information about a patient’s clinical history, family history, lifestyle, and current medications and micro-scale data about the cellular microenvironment, genetics and epigenetics of cells removed at a clinical exam. We use survival analysis models at present as a way to incorporate covariate information and predict cancer risk, but further refinement of mechanistic models is needed to make optimal use of the plentiful data being collected currently.
How can knowing an individual's risk of developing CRC be used to help in cancer prevention?
If we know a particular patient’s cancer risk, we can first create amenable ways of relaying that information to the patient, and then discussing viable treatment options, and weighing the risks versus benefits associated with those options. For patients with ulcerative colitis (UC), a form of IBD, we have developed a webtool called UC-CaRE that incorporates patient-specific information (lesion size, shape, etc.) to predict future cancer risk based on published data from previous patients from the same hospital. If the predicted risk is extremely high, this patient may opt to have their bowel removed. Although this procedure is life-altering, for some patients it will prevent a likely life-threatening cancer from developing, and knowing the chances of this increased risk will help patient/doctor shared-decision making.
Could modelling such as this be applied to other cancer types?
I believe this modelling can readily be applied to other cancer types, especially for those that we are finding increasing evidence that early stages of pre-cancer are detectable before a lethal cancer develops. The more quantitative discoveries we make about when and how patients’ cells change from pre-cancer to cancerous, the better we will be at finding those patients and intervening.
Dr Curtius’ work is supported by an MRC Rutherford Fund Research Fellowship.
Category: General News, Interviews

No comments yet