International study reveals four distinct types of oesophageal cancer
New research reveals that oesophageal squamous cell carcinoma (ESCC), one of the most aggressive and challenging cancers to treat, is composed of four distinct subtypes—each of which may benefit from different treatment approaches. This international collaboration between Barts Cancer Institute (BCI), Queen Mary University of London, and Zhengzhou University, in Henan Province, China, offers valuable insights into how ESCC interacts with and evades the immune system, potentially leading to more targeted therapies for patients.
Oesophageal cancer is one of the most challenging types of cancer to treat. It is often diagnosed at a late stage after it has already spread, when treatment options are limited. As a result, just 12% of patients with oesophageal cancer in England survive their disease for 10 years or more.
Worldwide, incidence and death from ESCC, the most common type of oesophageal cancer, are rising, with particularly high levels in Asia and Africa. Studying the populations most at risk of this cancer type could hold clues as to how to diagnose it earlier, treat it more effectively and improve survival in people with ESCC around the world.
Now, teams at the BCI and Zhengzhou University (working together as part of the Sino-British Research Centre for Molecular Oncology) have shed new light on ESCC in a new study published in Nature Communications.
The team gathered samples of tumour and normal tissue from 120 patients with ESCC in Henan Province – an area with one of the world’s highest incidences of this disease.
“This is one of the largest datasets of its kind from this region, with patients followed up for four years after surgery to remove their primary tumours,” explains Professor Yaohe Wang, who co-led the study at the BCI and Zhengzhou University.
Paving the way to more personalised treatment for oesophageal cancer
Analysing the genetic makeup of these tumours, the researchers identified four distinct subtypes of ESCC – ‘differentiated’, ‘immunogenic’, ‘metabolic’ and ‘stemness’ – each posing its own challenges in how it interacts with the immune system and responds to treatment. By understanding these subtypes, doctors may be able to offer more personalised treatments based on the specific characteristics of each patient’s tumour.
“The most hazardous subtype, which we term the ‘stemness’ subtype, is able to hide from the immune system, making it harder for the body to fight and potentially less susceptible to current immunotherapies,” Professor Wang explains. The team identified a gene (XCL1) that helps these tumours to evade immune attack and affects their sensitivity to common chemotherapy drugs. They also found that these tumours had high levels of a mutated gene (EP300) that could represent a promising target for new therapies.
By contrast, the team found that the ‘immunogenic’ subtype shows a much stronger immune response, suggesting that people with this type of ESCC may benefit from immunotherapies. Further studies will be crucial to confirm these findings and realise their potential in shaping patient care.
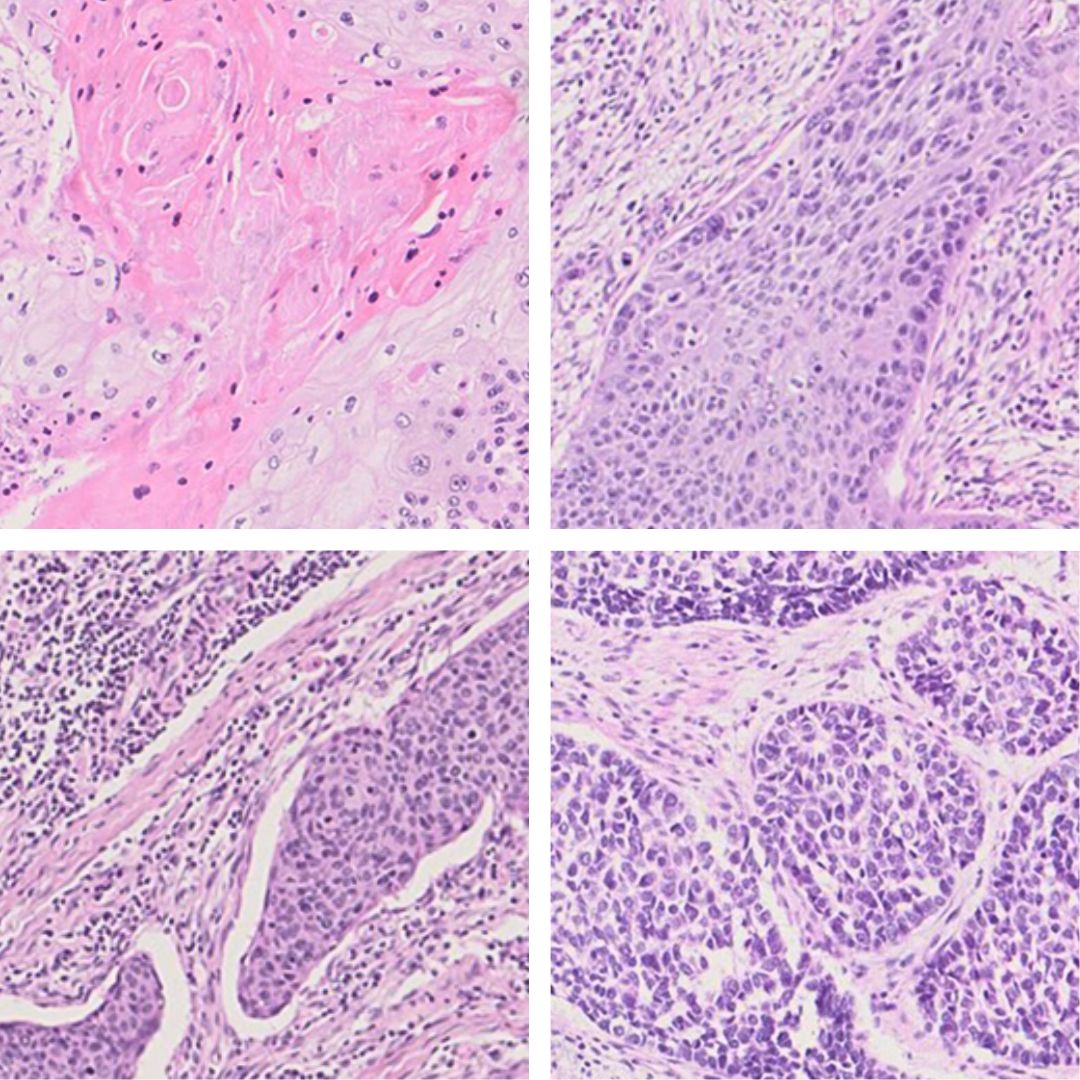
The team also developed an AI model to analyse microscope images of patient tissues and identify whether certain genetic subtypes of ESCC were linked to visible changes in the tissue. “We identified distinct imaging features specific to each subtype,” comments Dr Jun Wang, who co-led the study at the BCI. “We are now further developing and validating this model, to test whether specific features are correlated with clinical outcomes and molecular subtypes.”
Ultimately, this kind of AI tool could enable doctors to more quickly and accurately identify their patients’ disease subtypes and make more informed decisions about treatment.
This study provides vital new insights into our understanding of ESCC. Recognition of these four very different subtypes opens the door to more personalised approaches to selecting the best treatment for each patient’s disease. Further research is now needed, but ultimately, matching treatments to a person’s specific tumour type could provide much-needed improvements to survival for people with oesophageal cancer.
Category: General News, Publications

No comments yet