Skin cancer rewires its energy systems to spread more efficiently
Melanoma skin cancer cells radically rewire their internal power systems to drive their spread to other parts of the body, a new study shows. The research, led by investigators at Barts Cancer Institute, Queen Mary University of London, suggests that reversing this change can make tumour cells less invasive. The team also identifies a key molecule that orchestrates this process – knowledge that could lay the foundations for new therapeutic strategies to halt the spread of cancer.
Cancer cells’ ability to break away from the original tumour and spread to other parts of the body presents one of the greatest challenges to treating the disease. This process, called metastasis, seeds secondary tumours that grow in other organs, ultimately causing most cancer deaths.
“We’re still not targeting the secondary disease enough in the clinic, and I think we need to change this,” comments Professor Victoria Sanz-Moreno, corresponding author of the new study. “In our lab, we want to understand: what are the characteristics of cells that are able to metastasise? What are their weaknesses? And how do we target them?”
Melanoma skin cancer is among the quickest-spreading cancer types and is one of the focuses of Professor Sanz-Moreno and her laboratory’s research. If melanoma is diagnosed at an early stage before it spreads, almost all patients in the UK survive their disease for a year or more. But this survival drops to just over half once the disease has spread. The team’s work aims not only to equip us with the knowledge to better treat melanoma but also to unlock an improved understanding of how all cancers spread.
“We’re still not targeting the secondary disease enough in the clinic, and I think we need to change this.”
– Professor Victoria Sanz-Moreno
Building an efficient engine to power cancer spread
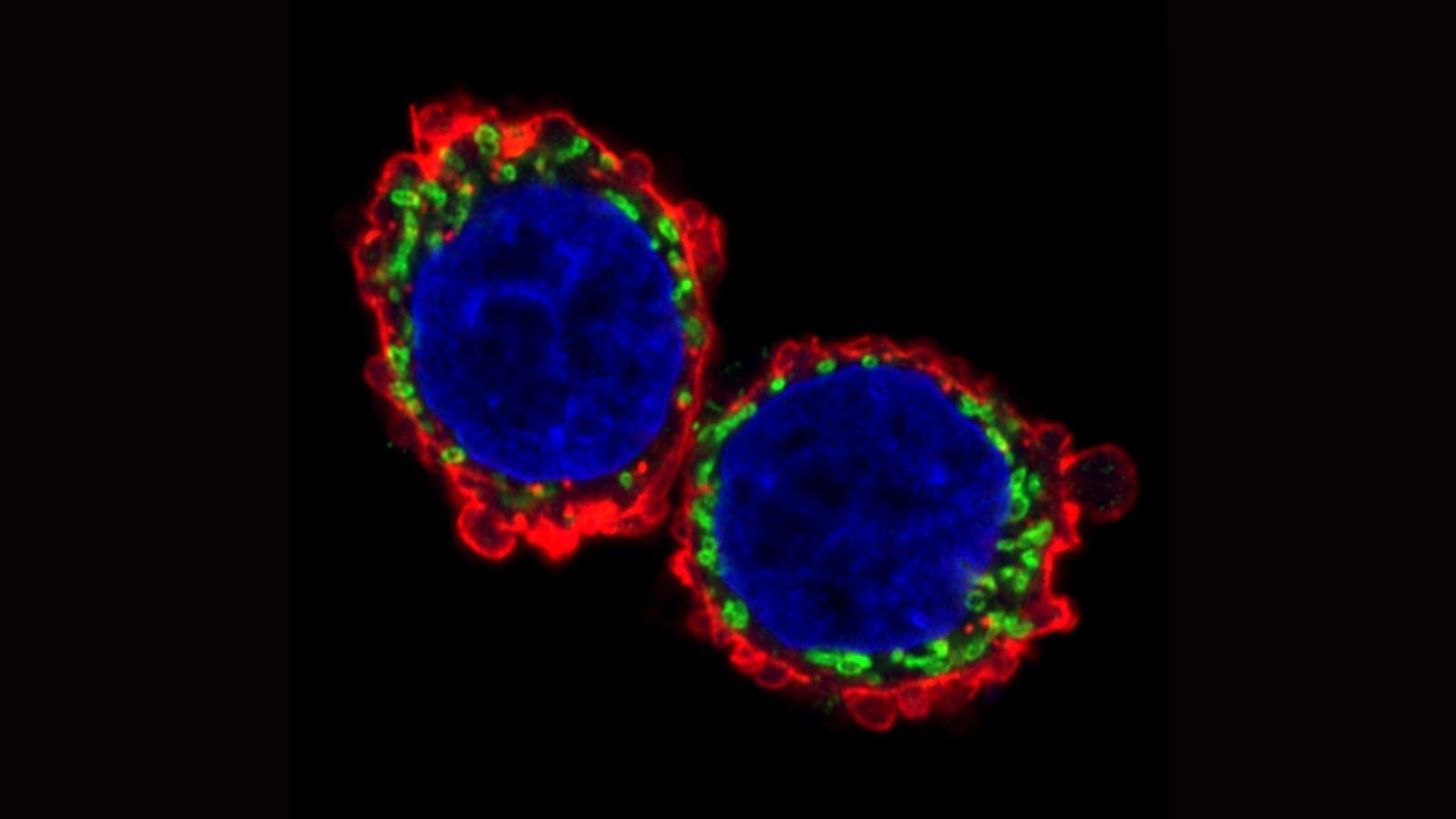
In the new study, published in Nature Communications, the team investigated how metastasising cells rewire their energy systems to move quickly and efficiently on their journey to other parts of the body. Two parts of the cell are key to this ability. Firstly, structures inside the cell called mitochondria – tiny power plants that produce chemical energy – fuel the cells’ movement. Secondly, cells’ internal skeleton – a network of fibres known as the cytoskeleton – must expand, contract and reshape to perform the movement.

The researchers examined migrating tumour cells in a special model system allowing movement in three dimensions – a departure from conventional systems that place cells on a flat surface that doesn’t accurately replicate how cells move through living tissue. They found that metastasising tumour cells adopt a style of movement known as rounded-amoeboid migration, where cells maintain a loose connection to their surroundings, enabling them to slither through the tissue. This requires far less energy than a common style of cell movement known as mesenchymal migration, where cells grip tightly onto their surroundings and drag themselves through their environment.
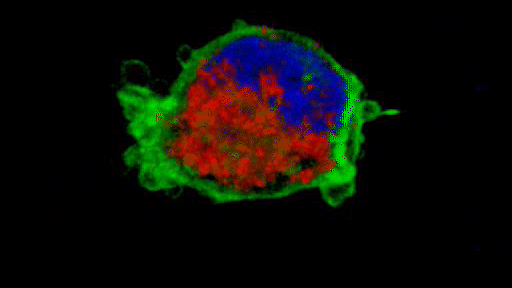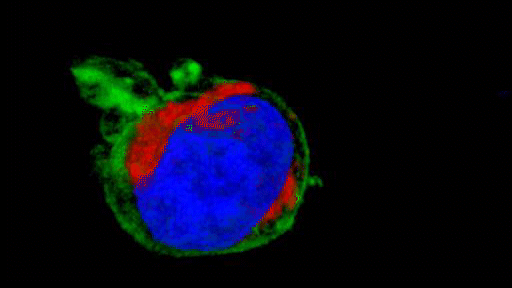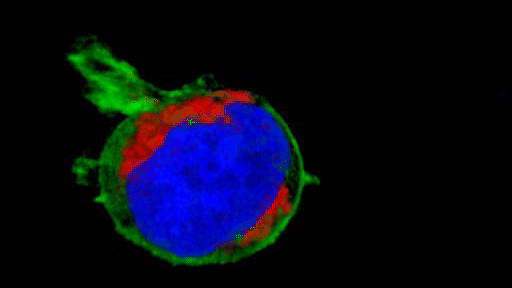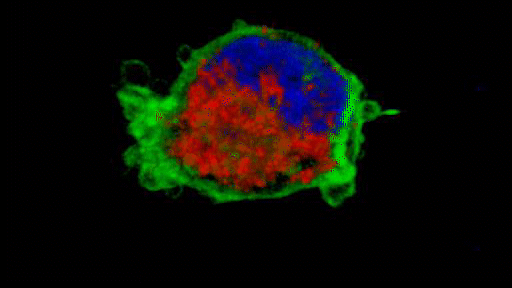
They observed that the invasive tumour cells reshape their mitochondria to suit this efficient style of movement, opting to have many, small, fragmented mitochondria that operate in a low-power mode. This is in contrast to less-invasive cells, which have large, branching networks of mitochondria that operate in a high-power mode.
“These metastatic cells are rewiring themselves to be very efficient,” explains Dr Eva Crosas-Molist, first author on the new paper. “They only need low levels of energy to move, which helps them to survive in the potentially stressful environments they are migrating to, where there may be a lack of nutrients or oxygen.”
Exposing a new weakness of metastasising cells
Intriguingly, the team found that if they manipulate the shape of the mitochondria in their metastasising tumour cells and force them to become more joined up, the cells lose their invasive behaviour. Likewise, if they make mitochondria more disconnected in less-invasive cells, the cells start to behave like metastasising tumour cells. The researchers discovered that a molecule called AMPK sits at the centre of these processes. It senses the energy requirements of the cell and also controls the cytoskeleton, which determines how the cell moves and behaves.
“That was a really surprising thing for us – we wouldn’t have imagined that changing the mitochondria could affect the cytoskeleton and vice versa.” Professor Sanz-Moreno explains. “By modifying these little mitochondria you create a global change, altering what the cell looks like and its whole behaviour.”
When the researchers examined migrating cells at the borders of tumours from humans, they saw the same type of changes in movement style and metabolism that they saw in their model, confirming that their observations reflect a real phenomenon in patients. What’s more, when they treated melanoma cells with a drug that blocks AMPK, it reduced their ability to the spread to the lung. This raises the possibility that targeting these pathways could present a promising new therapeutic strategy.
“This could be the beginning of us understanding a new set of metabolic vulnerabilities that we could exploit therapeutically in the future.”
– Professor Victoria Sanz-Moreno
“Molecules like AMPK that sit at the centre of a web of important processes involved in metastasis could present a weakness in cancer cells,” Professor Sanz-Moreno comments. “This could be the beginning of us understanding a new set of metabolic vulnerabilities that we could exploit therapeutically in the future.”
The work builds on previous findings from Professor Sanz-Moreno’s group earlier this year, which identified a protein that enables melanoma cells to deform their nucleus, enabling them to squeeze through small gaps and negotiate obstacles on their journey through the body. Better understanding how tumours cells rewire their internal components such as their nucleus, mitochondria and cytoskeleton to spread more rapidly could help us to develop new strategies to defend ourselves against these tactics and halt the spread of cancer.
Professor Ketan Patel, Chief Scientist at Cancer Research UK, a key funder of this work, said: “Patients whose cancer has spread often face tougher treatments and lower chances of survival. These insights about how cancer cells travel around the body could be incredibly valuable for designing interventions to prevent this in the future. The more we know about what’s happening in the bodies of people with cancer, the greater our ability to tackle it will be.”
The work was supported by Cancer Research UK; Barts Charity; Fundación Ramón Areces and World Wide Cancer Research
Category: General News, Publications

No comments yet