How cancer cells shapeshift to spread through the body
The spread of cancer from its starting point to other parts of the body – known as metastasis – is a critical obstacle to treating the disease. Metastasis is ultimately the cause of most cancer deaths, yet our ability to prevent it is limited. As such, we urgently need to understand how cells spread and find ways to stop them.
A new study published by Professor Susana Godinho and her team at the Barts Cancer Institute, Queen Mary University of London, has shed light on one way that cancer cells rearrange their inner workings to squeeze their way between obstacles – shapeshifting to escape their original tissue and travel through the body.

The new findings, published in EMBO Journal, focus on structures called centrosomes – small but crucial command centres that organise and sculpt cells’ internal skeleton, known as the cytoskeleton. This work was led by Dr Pedro Monteiro, Dr Bongwhan Yeon and Dr Sam Wallis in Professor Godinho’s group.

Transforming cells’ inner skeletons
The cytoskeleton has many important roles. It provides shape and structure to the cell, acts like a railway network to transport important cargo, and helps to separate the cell and its components during cell division, in addition to many other functions.
Cancer cells often develop an abnormally high number of centrosomes, which alters the structure of the cytoskeleton. This, in turn, disrupts processes such as cell division and results in cells with too many or too few copies of chromosomes, contributing to tumour development. Having too many centrosomes also seems to help cancer cells to invade other tissues, but it is not fully understood how. Professor Godinho and her team sought to shed light on this mystery in their new study.
“One key ability cells need in order to invade other tissues is to be able to deform their nucleus. Usually, the nucleus is quite stiff, but cells need to compress it in order to squeeze through tight gaps,” Professor Godinho explains. “We found that the nucleus becomes more deformable when cells have an increased number of centrosomes – the next step was to find out why.”
A radical reorganisation
Professor Godinho and her team found that when they stimulated cells to produce more centrosomes, it prompted a radical reorganisation of their contents. The cells transported their internal components along their cytoskeleton railway network to the outskirts of the cell.
This reorganisation included the transport of mitochondria, of the centrosomes themselves and of a molecule called vimentin, which normally helps to maintain the cell’s structure and forms a rigid cage around the nucleus. The transportation of vimentin away from the nucleus to the outskirts of the cell could help to explain why the nucleus becomes more supple and malleable in cancer cells. In support of this theory, Professor Godinho and the team observed that their cells with extra centrosomes did have a more deformable nucleus and were more able to squeeze themselves through small gaps.
The team found that these changes seemed to be driven by a chemical reaction called acetylation, which modifies particular molecules – such as protein called a-tubulin that makes up parts of the cytoskeleton – and impacts how the cytoskeleton transports cargo. Interestingly, the team found that the transportation of mitochondria and vimentin to the outskirts of the cell depended on the amount of tubulin that had undergone acetylation, which allowed cells to migrate through confined spaces. Surprisingly however, repositioning of the centrosome depended not only on increased tubulin acetylation but also on whether these acetylated tubulin filaments are distributed evenly or unevenly around the centrosome.
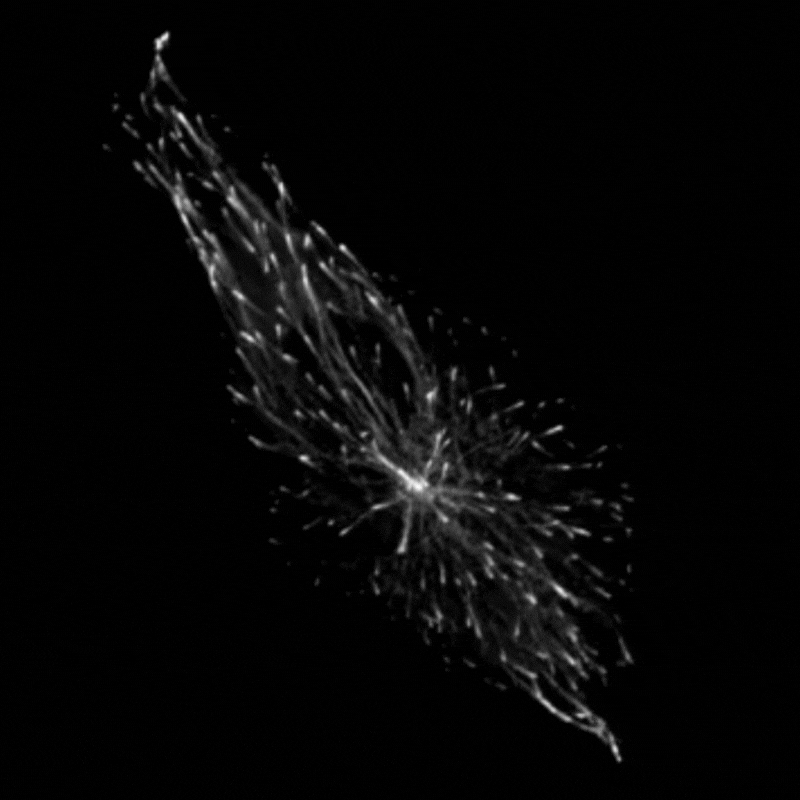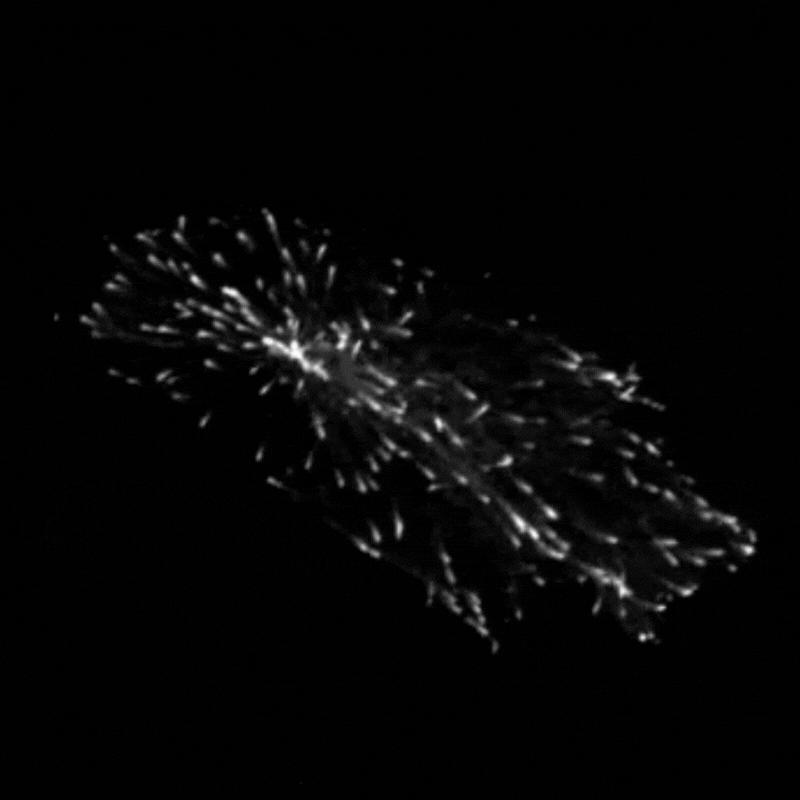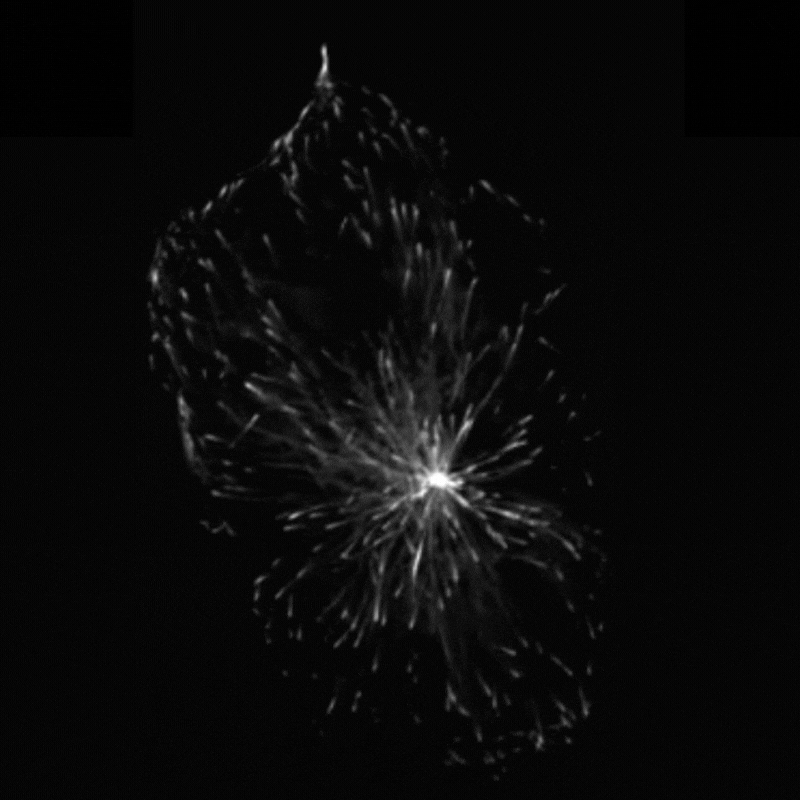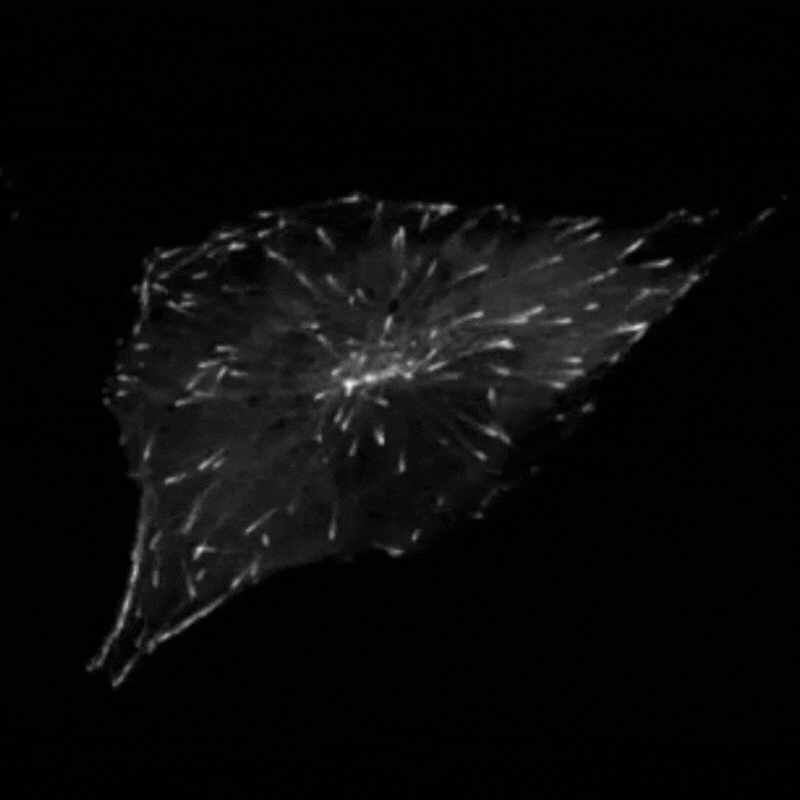
“We always assumed that only the amount of acetylation was important to affect transport,” Professor Godinho comments. “The fact that distribution had an effect was surprising to us – it’s something that has never been discussed before.”
Professor Godinho and her team found that when they stimulated cells to produce more centrosomes, it prompted a radical reorganisation of their contents. The cells transported their internal components along their cytoskeleton railway network to the outskirts of the cell.
Looking to the future
This new research not only helps us to understand how cancer spreads, but could also tell us something fundamental about how cells work, both in health and in disease. For example, cells in our immune system must migrate and squeeze through small gaps in order to reach an infection or other immune threat. Professor Godinho and her team are interested in investigating whether similar processes are at work in these healthy cells.
Professor Godinho also wants to dig deeper into the question of exactly how the cell nucleus changes when vimentin is taken away. Better understanding how cancer cells exploit this process to aid their spread could reveal critical steps that we can block with precise therapies in order to stop metastasis. While much more research is required, the possibilities for new treatment approaches are intriguing. “Can we prevent acetylation to block the efficient migration and spread of metastatic cells? I think that’s a really interesting idea,” Professor Godinho comments.
"Can we prevent acetylation to block the efficient migration and spread of metastatic cells? I think that’s a really interesting idea.”
— Professor Susana Godinho
This work was made possible thanks to funding from Cancer Research UK, Medical Research Council, and the Lister Institute of Preventive Medicine.
Category: General News, Publications

Ian Hart 24/08/2023
Another beautiful piece of work Susana. Well done indeed.