Research Focus
The focus of our lab is to study how inflammation, via modulating cellular metabolism, affects different aspects of cancer biology. In particular, we are interested in understating how obesity-associated inflammation rewires cellular metabolism, increasing the risk of breast cancer in obese women. We study (i) the initial phases of malignant transformation, (ii) the contribution of different immune cells (i.e. DCs and T cells) and (iii) drug resistance mechanisms.
Key Publications
The breast cancer oncogene IKKε coordinates mitochondrial function and serine metabolism. EMBO Rep (2020) 21(9):e48260. PMID: 32783398
Macrophages induce malignant traits in mammary epithelium via IKKε/TBK1 kinases and the serine biosynthesis pathway. EMBO Mol Med (2020) 12(2):e10491. PMID: 31930708
RIPK1 and Caspase-8 Ensure Chromosome Stability Independently of Their Role in Cell Death and Inflammation. Mol Cell (2019) 73(3):413-428.e7. PMID: 30598363
The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell (2011) 43(3):432-48. PMID: 21737329
Major Funding
- 2023-2025- Barts Charity, Large Project Grant, 'Barts Metabolism Network - Inhibiting the serine metabolism pathway to lower the risk of breast cancer in obese women,' £497,000
- 2022-2026- MRC DTP, iCASE studentship in collaboration with Achilles Therapeutics, 'Characterizing the metabolic requirements of the newly identified dendritic cell subset DC3'
- 2021-2025- BBSRC, LiDO iCASE studentship in collaboration with AstraZeneca, 'Understanding cancer cell dependency on ATR/ATM: a metabolism or DNA repair tail?' £112,000
- 2021-2022- Medcity, Collaborate to Innovate: Advanced Therapies in collaboration with Dr Paula Longhi and Achilles Therapeutics, £49,924
Other Activities
- Founder of The London Inflammation Network
- Manager of the facility for metabolic flux analysis of the Faculty of Medicine and Dentistry, Queen Mary University of London
- Academic lead for the MRC-DTP in Translational Immunology, Inflammation and Cancer
- Co-module lead of the course for Cancer Biology, 3rd year BSc (Queen Mary)
- Member of the Barts Metabolism Network
Research
Cellular metabolism supports every cellular function from proliferation to survival and differentiation. This is true for cancer cells, where alterations in metabolism have been described as hallmarks of cancer, but also in every cell type, including immune cells.
Current projects:
1. Investigating the effects of adipose tissue on the cell intrinsic metabolism of conventional dendritic cells
(Led by Robert Hearnden, in collaboration with MP Longhi - WHRI)
Conventional dendritic cells (cDCs) are crucial cell-mediators of immunity at the interface between the innate and adaptive immune systems. Both major subsets of cDC, cDC1 and cDC2, have important roles in promoting tolerance during the steady-state and inflammatory responses when challenged. As the immune system’s professional antigen presenting cells, cDCs occupy most tissues in the body, and can migrate through the lymphatics to promote the required immune response. This means that cDCs have a vast range of functional plasticity and are exposed to several metabolic environments within and between tissues. Even though each cDC cellular process is underpinned by cell intrinsic metabolism, very little is known about how different metabolic landscapes influence cDC metabolism and subsequently their function. Chronic inflammation within the adipose tissue (AT) during obesity has been proposed as a common process in the pathophysiology of obesity associated co-morbidities such as diabetes and cancer. AT cDCs have been shown to contribute to this development. In this project we use metabolic assays and metabolomic approaches in ex vivo and in vitro cDCs to show that AT cDCs are metabolically distinct from cDCs in other tissues.
2. Characterizing the metabolic requirements of the newly identified dendritic cell subset DC3
(Led by Emily Golding, in collaboration with MP Longhi (WHRI) and Pablo Becker (Achilles Therapeutics)).
Following up from the previous project, where we have demonstrated that the metabolism of cDC1 and cDC2 is differentially regulated to support their different functions, here we are investigating the metabolism of DC3 and its functional implications. Three subtypes of DC have been identified so far: (i) classical DC1 (cDC1) known for their ability to stimulate CD8+ T-cell responses; (ii) cDC2, activator of CD4+ T-cells and (iii) the recently identified DC3, that play a central role in tumour immune-surveillance by priming tissue-resident T-cells.
3. Phenocopying ATM loss via modulation of cellular metabolism to overcome resistance to the ATR inhibitor
(Led by Hannah Rouse, in collaboration with AZ)
AZD6738 is an ATR inhibitor (clinical trials - NCT03682289, NCT04564027, NCT03334617) that demonstrated a dose-dependent tumour growth inhibition when used as monotherapy mainly in ATM-deficient xenograft models, suggesting a role for ATM in inducing drug resistance. While this ascertains that in patients with ATM loss/mutations the drug is more effective, on the other side it also represents a major limitation to its utilization. Thus, understanding how ATM mediates resistance to an ATR inhibitor is of major interest. Our hypothesis is that metabolic changes linked to ATM deficiency lead to drug resistance.
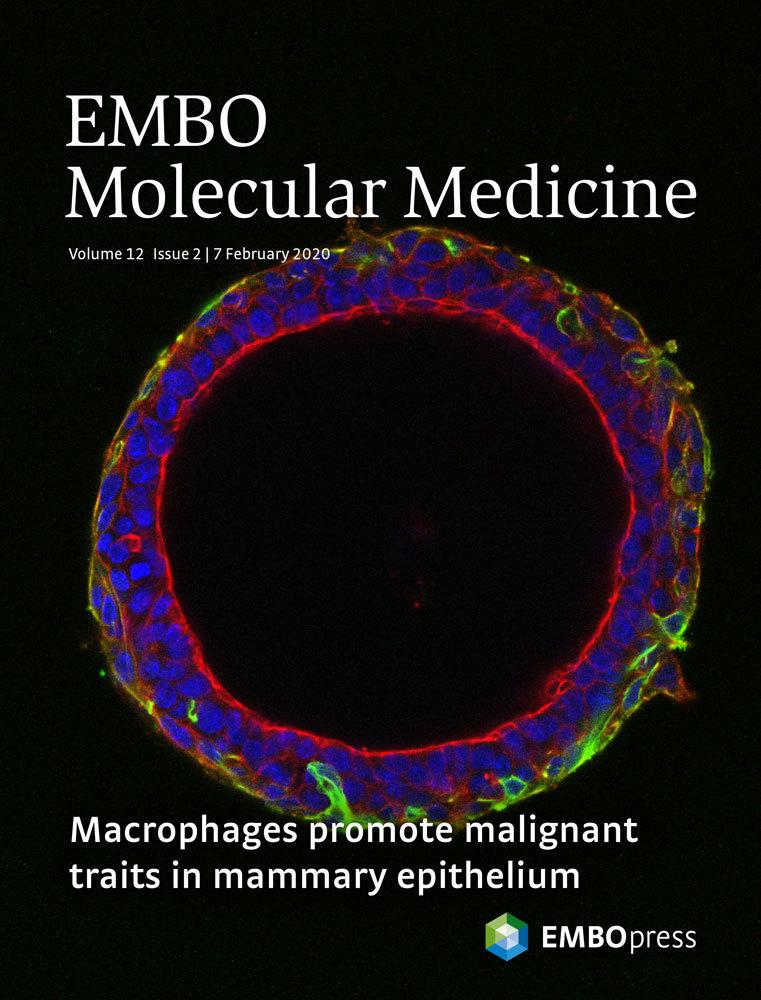
4. Inhibiting the serine biosynthesis pathway to lower the risk of breast cancer in obese women
(Currently recruiting PDRA, project part of the Barts Metabolism Network)
Chronic inflammation is the underlying cause of major pathologies and is considered the main factor responsible for higher risk of developing breast cancer in obese women. We have demonstrated that activation of the serine biosynthesis pathway (SBP) by IKKepsilon, a key mediator of the innate immune response and a breast cancer oncogene, is a causal link to malignant transformation (Xu R et al, EMBO Reports 2020 and Wilcz-Villega E EMBO Mol Med 2020). Importantly, both IKKepsilon and the SBP are upregulated in adipocytes in obesity. Here we will investigate the functional role of the SBP in adipocytes during obesity and its contribution to breast cancer development.
The final scope of our research is to design better combination therapy to limit inflammation driven malignant transformation, possibly combining anti-inflammatory drugs with drugs targeting different metabolic pathways.
We also run the Barts and the London School of Medicine and Dentistry facility for metabolic flux analysis. Dr Valle Morales is our Mass Spectrometry specialist.
Other Activities
- Founder of The London Inflammation Network- Bringing together the wider community of scientists working on inflammation, an exciting opportunity for junior investigators to present their work and network.
- Manager of the facility for metabolic flux analysis of the Faculty of Medicine and Dentistry, Queen Mary University of London
- Academic lead for the MRC-DTP in Translational Immunology, Inflammation and Cancer
- Co-module lead of the course for Cancer Biology, 3rd year BSc (Queen Mary)
- Member of the Barts Metabolism Network
Major Funding
- 2023-2025- Barts Charity, Large Project Grant, 'Barts Metabolism Network - Inhibiting the serine metabolism pathway to lower the risk of breast cancer in obese women,' £497,000
- 2022-2026- MRC DTP, iCASE studentship in collaboration with Achilles Therapeutics, 'Characterizing the metabolic requirements of the newly identified dendritic cell subset DC3'
- 2021-2025- BBSRC, LiDO iCASE studentship in collaboration with AstraZeneca, 'Understanding cancer cell dependency on ATR/ATM: a metabolism or DNA repair tail?' £112,000
- 2021-2022- Medcity, Collaborate to Innovate: Advanced Therapies in collaboration with Dr Paula Longhi and Achilles Therapeutics, £49,924
- 2019-2021- MRC DTP programme, 'Investigating the cell intrinsic metabolism of conventional dendritic cells and identifying whether this is altered in adipose tissue
- 2018-2021- Barts Charity Project grant, 'Creating a facility for metabolic flux analysis,' £497,691 + £50,000
- 2016-2018- Cancer Research UK Pioneer Award, 'Reducing intracellular serine concentration for cancer treatment,' £182,977
Recent Publications
The Glucose Transporter 2 regulates CD8+ T cell function via environment sensing. Fu H, Vuononvirta J, Fanti S et al. Nature Metabolism (2023) (1)
Vitamin B5 and succinyl-CoA improve ineffective erythropoiesis in SF3B1-mutated myelodysplasia Mian SA, Philippe C, Maniati E et al. Science Translational Medicine (2023) 15(10) eabn5135-eabn5135
Loss of hydrogen voltage-gated channel-1 expression reveals heterogeneous metabolic adaptation to intracellular acidification by T-cells Coe D, Poobalasingam T, Fu H et al. JCI Insight (2022) 7(10)
Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy Fernandez-Caggiano M, Kamynina A, Francois AA et al. Nature Metabolism (2020) 2(10) 1223-1231
The breast cancer oncogene IKKε coordinates mitochondrial function and serine metabolism Xu R, Jones W, Wilcz‐Villega E et al. EMBO Reports (2020) 21(10) e48260
Macrophages induce malignant traits in mammary epithelium via IKKε/TBK1 kinases and the serine biosynthesis pathway Wilcz‐Villega E, Carter E, Ironside A et al. EMBO Molecular Medicine (2020) 12(10) e10491
RIPK1 and Caspase-8 Ensure Chromosome Stability Independently of Their Role in Cell Death and Inflammation Liccardi G, Garcia LR, Tenev T et al. Molecular Cell (2019) 73(10) 413-428.e7
Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2 Annibaldi A, John SW, Berghe TV et al. Molecular Cell (2018) 69(10) 566-580.e5
Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018 Galluzzi L, Vitale I, Aaronson SA et al. Cell Death & Differentiation (2018) 25(10) 486-541
The unconventional myosin CRINKLED and its mammalian orthologue MYO7A regulate caspases in their signalling roles Orme MH, Liccardi G, Moderau N et al. Nature Communications 7(10) 10972
For additional publications, please click hereTeam
Present:
- Dr Valle Morales, Mass Spectrometry specialist
- Mr Robert James Hearnden, MRC-DTP PhD student
- Mrs Hannah Rouse, BBSRC-LiDO iCASE student, collaboration with AZ
- Mrs Emily Golding, MRC-DTP iCASE student, collaboration with Achilles Therapeutics

Recruiting:
- Postdoctoral Research Assistant (PDRA)
- Technician
Past members:
- Dr Ruoyan Xu, PDRA
- Dr Ewa Wilcz-Villega, PDRA
- Dr William Jones, PhD student
- Dr Isabella Mataloni, PhD student
Biography
I obtained my PhD in Molecular Medicine (2006) in Ferrara, Italy, studying the role of mitochondrial signalling and metabolism in different pathophysiological conditions, under the supervision of Prof. Rosario Rizzuto. I then moved for a short postdoctoral period to the INSERM in Paris, to study the role of the mitochondria in hepatocellular tumour formation.
In 2007 I obtained a FEBS long-term postdoctoral fellowship to join the apoptosis laboratory at the Institute of Cancer Research, London, led by Prof. Pascal Meier. During my post-doctoral training, I focused on the family of protein IAPs (Inhibitor of Apoptosis) and their role in inflammation and cancer. As their name suggests, this family of proteins has a strong anti-apoptotic role and we studied their role in chemotherapy-induced death of cancer cells. Thus we identified the Ripoptosome, a newly described protein complex, which plays a crucial role in cell death induction, both via apoptosis and necroptosis.

