
Dr Oliver M. Pearce
BSc (Hons), PhD
Reader in Tumour Extracellular Matrix
Group Leader
Research Focus
The focus of our research is the tumour microenvironment (TME). We are particularly interested in understanding the composition and function of the tumour extracellular matrix in immunosuppression.
Key Publications
Deconstructing a metastatic human tumor microenvironment. Cancer Disc (2018) 3, 304-319. PMID: 29196464
Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res (2017) 16, 3083-3091. PMID: 28675934
A red meat-derived glycan promotes inflammation and cancer progression. PNAS (2015) 112, 542-547. PMID: 25548184
Engagement of myelomonocytic Siglecs by tumor-associated ligands regulates innate immune responses to cancer. PNAS (2014) 111, 14211-14216. PMID: 25225409
Major Funding
- 2019-2025- Cancer Research UK, ‘Restoring response to immunotherapy by targeting the extracellular matrix’,' £813,040
- 2018-2019- Against Breast Cancer, Characterising the glycan shield of the extracellular matrix
Other Activities
- Member of the 'British Society for Matrix Biology'
Research
What we do
We are investigating the composition and function of the tumour extracellular matrix, which has been identified as a barrier to immunotherapy response. Our overall goal is to target this barrier to improve patient response rates to immunotherapy. Towards this goal we are characterizing the tumour extracellular matrix and developing 3D tumour models for functional studies. The lab uses a combination of molecular biology, biochemistry, and genetic engineering approaches.
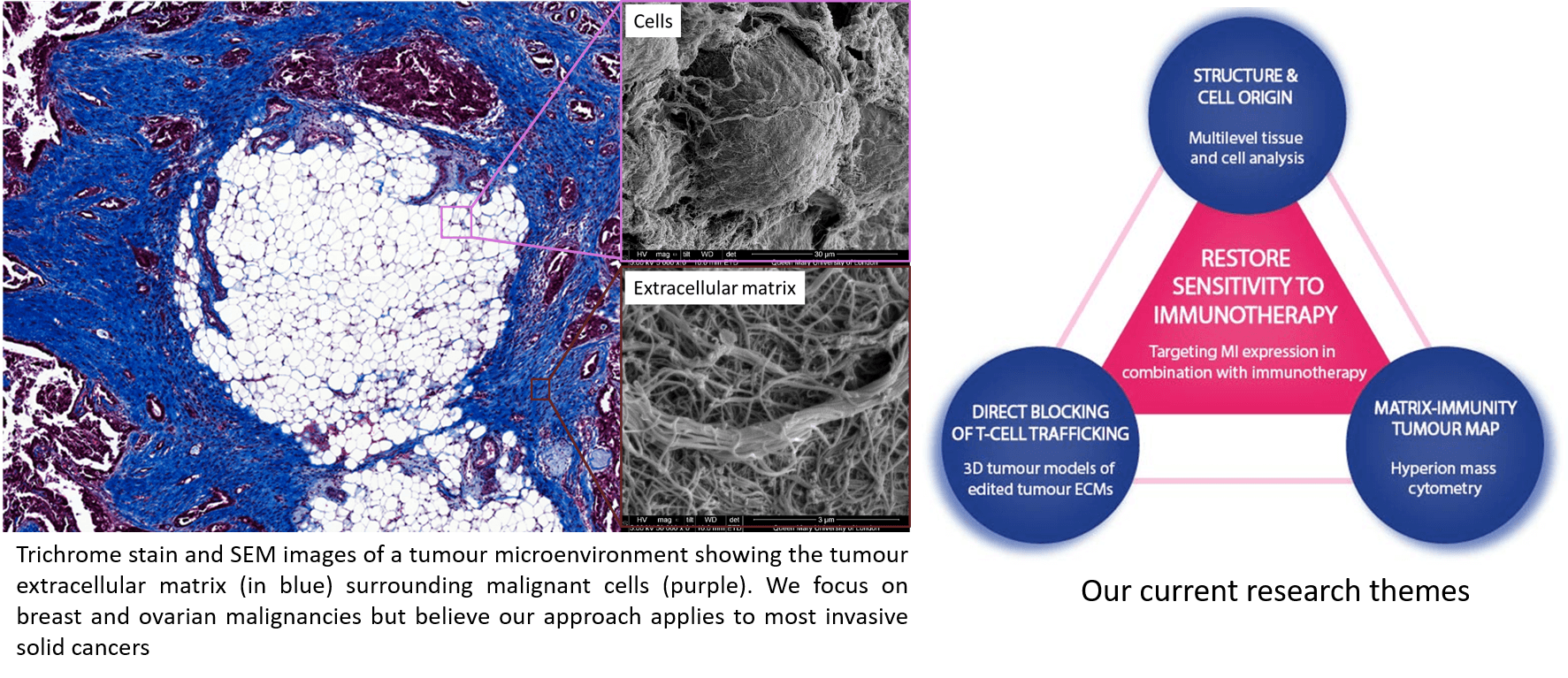
Our methods and technology
To study the tumour microenvironment, our lab has developed some methodology and technology that is unique to our lab.
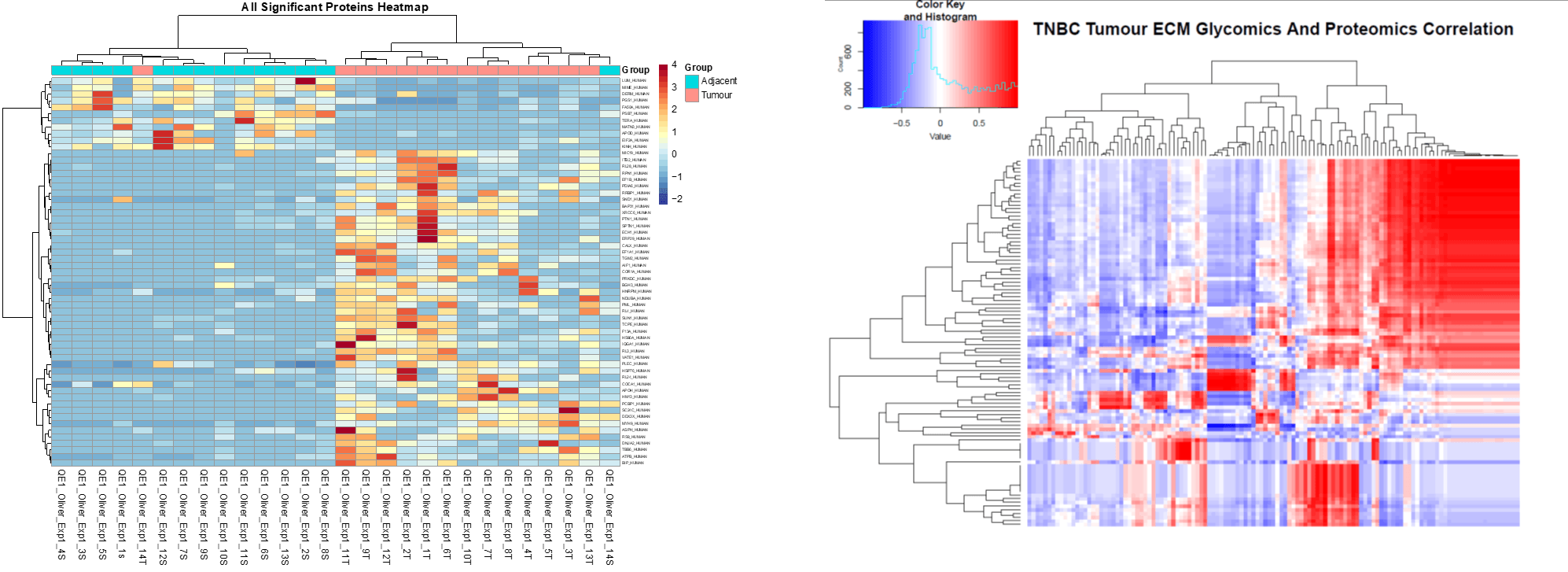
Matrisome proteomics and glycomics. Up until quite recently it was difficult to characterise the tissue composition of tumour tissues. We use a matrisome proteomics approach to characterise the composition of tumour tissue at the translational (protein) level and the post-translational (glycan) level. We use these types of information to identify potential molecules within the tumour microenvironment that might play an active role in disease progression.
Engineered 3D tumour microenvironments. To test the role of our target molecules we use several 3D in vitro tumour models made from primary human cancer tissues. These provide a physiologically relevant and semi high throughput platform to exploring tumour progression, tumour immunity, and emerging biological therapies.

Bioinformatics. We use informatic approaches to analyze our characterization data sets. In particular we integrate protein and glycan data as a way to identify highly disease specific markers called ‘glycoforms’.
Current research projects:
- Research Project 1: The role of the tumour matrix on immune cell activation
In our previous studies, we have deconstructed the TME of high grade serous ovarian cancer (HGSC) and identified twenty-two ECM molecules that we use as a quantified ratio or 'matrix index', MI, found to be common in many human carcinomas (Pearce, Delaine-Smith, Maniati, et al. 2018). A major finding from this work has been the strong correlation of the MI with immunosuppressive immune cell phenotypes and poor prognosis in solid tumour patients. Understanding how the ECM disrupts anti-tumour immunity and regulates the local immune environment could identify new targets to treat many cancers. Our hypothesis is, components of the MI communicate with tumour infiltrating immune cells to generate immunosuppressive phenotypes. This work is being done by Elliott Puttock, in collaboration with Dr. Ann White at UCB Pharma.
- Research Project 2: Characterising the glycan shield of the tumour extracellular matrix
Complementary to project 1, the aim of this work is to further investigate the MI (described above) in triple negative breast cancer tissues, including characterising the post-translational modifications on MI proteins, which we think are important in generating the immunosuppressive TME. This work is being done by Ying Liu and Priyanka Hirani in my lab, and in collaboration with Dr. Alexandra Naba (University of Illinois, Chicago), Dr. Pedro Cutillas (QMUL), and Dr. Stuart Haslam (Imperial College London).
- Research Project 3: Investigating a specific Matrix Index molecule in forming an immune-barrier within the tumour microenvironment.
As a result of research project 2, we have identified one particular matrix index molecule that associates with inhibition of cytotoxic T-cell infiltration within the tumour microenvironment. Taking a biochemical approach we are following up on this observation to characterise the proteoglycans structure and immunological function. This work is being done by Priyanka Hirani in my lab, in collaboration with Dr. Pedro Cutillas (BCI), and Prof. Tom Wight (Benaroya Research Institute, Seattle).
Other Activities
- Member of the 'British Society for Matrix Biology'
Major Funding
Recent Publications
A Novel Primary Cilium‐Mediated Mechanism Through which Osteocytes Regulate Metastatic Behavior of Both Breast and Prostate Cancer Cells Verbruggen SW, Nolan J, Duffy MP et al. Advanced Science (2024) 11(10) 2305842
Opposing roles for ADAMTS2 and ADAMTS14 in myofibroblast differentiation and function Carter EP, Yoneten KK, Gavara N et al. The Journal of Pathology (2024) 262(10) 90-104
Opposing roles for ADAMTS2 and ADAMTS14 in myofibroblast differentiation and function Carter E, Yoneten K, Gavara N et al. Pancreatology (2023) 23(10) e13-e14
Extracellular matrix educates an immunoregulatory tumor macrophage phenotype found in ovarian cancer metastasis Puttock EH, Tyler EJ, Manni M et al. Nature Communications 14(10) 2514
Abstract 1339: Targeting specific extracellular matrix proteins to reactivate T cell trafficking in triple-negative breast cancer Gauthier V, Tyler E, Liu Y et al. Cancer Research (2023) 83(10) 1339-1339
Abstract 5841: Exploring the role of versican in immune exclusion within triple negative breast cancer Hirani P, Alonge KM, Pennington DJ et al. Cancer Research (2023) 83(10) 5841-5841
Organ-on-a-Chip and Microfluidic Platforms for Oncology in the UK Nolan J, Pearce OMT, Screen HRC et al. Cancers 15(10) 635
YAP activation inhibits inflammatory signalling and cartilage breakdown associated with reduced primary cilia expression Meng H, Fu S, Ferreira MB et al. Osteoarthritis and Cartilage (2022) 31(1) 600-612
Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade Stanczak MA, Rodrigues Mantuano N, Kirchhammer N et al. Science Translational Medicine (2022) 14(10) eabj1270-eabj1270
Editorial: Glycans: Masters of immunity, from cancers to inflammatory disease Beatson R, Läubli H, Pearce OMT et al. Frontiers in Immunology 13(10) 1002679
For additional publications, please click hereTeam
Postdoctoral Researchers
Dr Elly Tyler
PhD Students
Mr Elliott Puttock, Ms Ying Liu, Ms Priyanka Hirani, Ms Valentine Gauthier
Alumni
Biography
I originally trained as an organic chemist with Prof Ben Davis and Prof Len Seymour at Oxford University. My PhD thesis was on the development of chemically glycosylated viral vectors for cancer gene therapy.
For my post-doctoral studies I moved to Prof. Ajit Varki’s lab at the University of California, San Diego. Here I investigated how glycans are involved in cancer immunity. After five years in California, I returned to the UK for a second post-doc with Prof. Fran Balkwill at Barts Cancer Institute to further train in cancer biology.
In September 2017 I started my own research program with funding from UCB Pharma and Against Breast Cancer. The theme of our work is the tumour matrisome and its role in tumour immunity.
